Background:
Allogeneic HCT continues to be the optimal consolidation strategy for many patients with AML. Cytogenetic and molecular abnormalities are known to influence post-transplant outcomes. We tested the prognostic ability of a modified (mELN) classification system based on available CIBMTR genetic data, to predict post-transplant outcomes.
Methods:
Adult patients with a diagnosis of AML in first complete remission (CR1) with available pre-transplant cytogenetic and molecular mutational data, receiving a first allogeneic HCT from 2013-2017, were included. Patients were stratified according to mELN genetic classification in three distinct groups: favorable (Fav), intermediate (IM) and adverse (Adv). Clinical outcomes following HCT were compared among groups after adjusting for significant patient, disease, and transplant-related variables. The primary endpoint was disease free survival (DFS). Secondary endpoints were overall survival (OS), non-relapse mortality (NRM), cumulative incidence of relapse, acute GVHD, and chronic GVHD. Cox proportional hazard models were used to compare endpoints among mELN risk groups, age groups and genetic subsets within the adverse-risk group.
Results:
Demographic characteristics are summarized in Table 1. 2289 patients (Fav, n=181; IM n=1185; and Adv n=923) met the inclusion criteria. Median follow-up for survivors was 35 months. Importantly, 41% of transplant recipients (n=936) were >60 years, 76% (n=1743) had de novo AML and 48% (n=1111) received a myeloablative conditioning regimen.
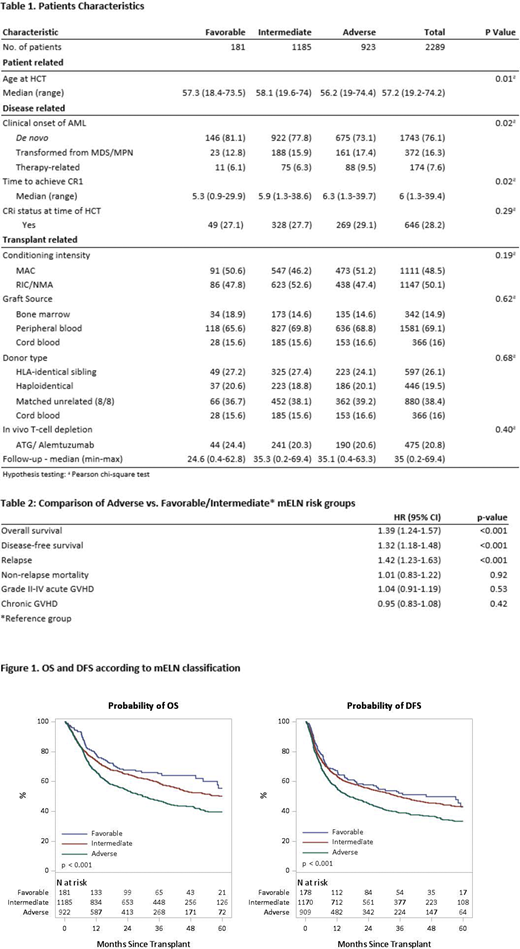
Univariate analysis (UVA) demonstrated significant differences in 2-year OS (Fav: 67.7%, IM: 64.9% and Adv: 53.9%; p<0.001); DFS (Fav: 57.8%, IM: 55.5% and Adv: 45.3; p<0.001) and relapse (Fav: 28%, IM: 27.5% and Adv: 37.5%; p<0.001) There were no significant differences in NRM (p=0.467) or the incidence of acute (p=0.423) and chronic GVHD (p=0.442) among mELN groups.
Initial multivariate analysis (MVA) of mELN risk groups indicated that there was no significant difference in clinical outcomes between the Fav and IM risk groups. Thus, these groups were combined for subsequent analyses. Adv risk (vs. Fav/IM) led to significantly worse OS (HR 1.39 [1.24-1.57] p=<0.001), DFS (HR 1.32 [1.18-1.48] p=<0.001), and relapse (HR 1.42 [1.23-1.63] p=<0.001) (Table 2).
This mELN classification effectively stratified both younger (<60 y/o) and older (>60 y/o) patients for OS (Adv vs. Fav/IM HR for <60: 1.43 [1.21-1.69] p<0.001; >60: 1.44 [1.21-1.72] p<0.001), DFS (Adv HR for <60: 1.31 [1.12-1.53] p<0.001; >60: 1.41 [1.19-1.66] p<0.001) and relapse (Adv HR for <60: 1.44 [1.20-1.74] p<0.001, >60: 1.42 [1.15-1.76] p=0.001). NRM was higher for older patients (>60 y/o) in both the Fav/IM (HR 1.40 [1.10-1.78] p=0.007) and Adv-risk cohorts (HR 1.59 [1.18-2.13] p=0.002). Genetic subset comparisons within the adverse-risk group showed that patients carrying monosomy 5, del(5q) or monosomy 7 had inferior 2-year OS (42.6%, p<0.001) and DFS (35.2%, p<0.001), as well as higher rates of relapse (45%, p= 0.002) when compared to other patients within the Adv-risk cohort (OS 60%, DFS 50.8%, relapse 33.5%).
Conclusion:
Stratification using mELN criteria resulted in clear prognostic separation of OS, DFS and relapse in this large cohort of AML patients undergoing allogeneic HCT. While Fav and IM groups had similar OS, DFS and relapse rates; patients in the Adv risk group had the highest risk of relapse and inferior DFS/OS. However, the majority of patients in all cohorts had favorable outcomes for HCT in CR1.
Our findings confirm the value of a combined genetic prognostic model in the AML HCT setting and justify the use of this stratification system in future HCT trials. Correlation of genetic subtypes with other important transplant variables, such as conditioning intensity and pre-transplant MRD status deserves further evaluation. There remains a subset of Adv-risk patients for which post-transplant outcomes continue to be poor, even when transplanted in CR1. Novel peri-transplant pre-emptive/therapeutic strategies are urgently needed for this high-risk cohort.
de Lima:Celgene: Research Funding; Pfizer: Other: Personal fees, advisory board, Research Funding; BMS: Other: Personal Fees, advisory board; Incyte: Other: Personal Fees, advisory board; Kadmon: Other: Personal Fees, Advisory board. Komanduri:Kiadis: Consultancy; Takeda: Consultancy; Celgene: Consultancy; Atara: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kebriaei:Ziopharm: Other: Research Support; Jazz: Consultancy; Kite: Other: Served on advisory board; Amgen: Other: Research Support; Novartis: Other: Served on advisory board; Pfizer: Other: Served on advisory board. Bhatt:National Marrow Donor Program: Research Funding; Rigel Pharmaceuticals: Other; Jazz: Research Funding; Agios: Other: Personal Fees; Incyte: Other: Personal Fees, Research Funding; Takeda: Other: Personal Fees; Partner Therapeutics: Other: Personal Fees; Pfizer: Other, Research Funding; CSL Behring: Other; Tolero Pharmaceuticals: Research Funding; Oncoceutics: Other: Drug support for a trial; Partnership for health analytic research, LLC: Other: Personal Fees; Omeros: Other: Personal Fees; Abbvie: Other: Personal Fees, Research Funding. Saad:Orcabio: Other: research support; Kadmon: Other: research support; Amgen: Other: research support; Incyte Pharmaceuticals: Other: Personal Fees; Magenta Therapeutics: Other: Personal Fees. Copelan:Amgen: Membership on an entity's Board of Directors or advisory committees. Defilipp:Incyte: Research Funding; Regimmune: Research Funding; Syndax Pharmaceuticals: Consultancy. Nishihori:Karyopharm: Other: Research support to institution; Novartis: Other: Research support to institution. Weisdorf:Incyte: Research Funding; FATE Therapeutics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.